Page 198 - DMGT401Business Environment
P. 198
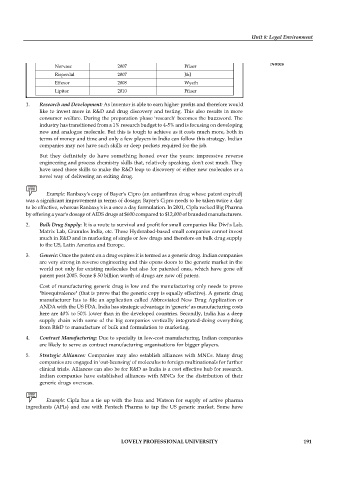
Drug Year Innovator
Zitromax 2005 Pfizer
Prevacid 2006 Takeda
Zocor 2006 Merck
Unit 8: Legal Environment
Pravachol 2006 Bristol Myers Squibb
Zoloft 2006 Pfizer
Paxil 2006 GlaxoSmithkline
Notes
Norvasc 2007 Pfizer
Risperdal 2007 J&J
Effexor 2008 Wyeth
Lipitor 2010 Pfizer
1. Research and Development: As inventor is able to earn higher profits and therefore would
like to invest more in R&D and drug discovery and testing. This also results in more
consumer welfare. During the preparation phase 'research' becomes the buzzword. The
industry has transitioned from a 1% research budget to 4-5% and is focusing on developing
new and analogue molecule. But this is tough to achieve as it costs much more, both in
terms of money and time and only a few players in India can follow this strategy. Indian
companies may not have such skills or deep pockets required for the job.
But they definitely do have something honed over the years: impressive reverse
engineering and process chemistry skills that, relatively speaking, don't cost much. They
have used those skills to make the R&D leap to discovery of either new molecules or a
novel way of delivering an exiting drug.
Example: Ranbaxy's copy of Bayer's Cipro (an antianthrax drug whose patent expired)
was a significant improvement in terms of dosage; Bayer's Cipro needs to be taken twice a day
to be effective, whereas Ranbaxy's is a once a day formulation. In 2001, Cipla rocked Big Pharma
by offering a year's dosage of AIDS drugs at $600 compared to $12,000 of branded manufacturers.
2. Bulk Drug Supply: It is a route to survival and profit for small companies like Divi's Lab,
Matrix Lab, Granules India, etc. These Hyderabad-based small companies cannot invest
much in R&D and in marketing of single or few drugs and therefore on bulk drug supply
to the US, Latin America and Europe.
3. Generic: Once the patent on a drug expires it is termed as a generic drug. Indian companies
are very strong in reverse engineering and this opens doors to the generic market in the
world not only for existing molecules but also for patented ones, which have gone off
patent post 2005. Some $ 50 billion worth of drugs are now off patent.
Cost of manufacturing generic drug is low and the manufacturing only needs to prove
"bioequivalence" (that is prove that the generic copy is equally effective). A generic drug
manufacturer has to file an application called Abbreviated New Drug Application or
ANDA with the US FDA. India has strategic advantage in 'generic' as manufacturing costs
here are 40% to 50% lower than in the developed countries. Secondly, India has a deep
supply chain with some of the big companies vertically integrated-doing everything
from R&D to manufacture of bulk and formulation to marketing.
4. Contract Manufacturing: Due to specialty in low-cost manufacturing, Indian companies
are likely to serve as contract manufacturing organisations for bigger players.
5. Strategic Alliances: Companies may also establish alliances with MNCs. Many drug
companies are engaged in 'out-licensing' of molecules to foreign multinationals for further
clinical trials. Alliances can also be for R&D as India is a cost effective hub for research.
Indian companies have established alliances with MNCs for the distribution of their
generic drugs overseas.
Example: Cipla has a tie up with the Ivax and Watson for supply of active pharma
ingredients (APIs) and one with Pentech Pharma to tap the US generic market. Some have
LOVELY PROFESSIONAL UNIVERSITY 191